Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakajima, Kunihisa; Nakazono, Yoshihisa; Arai, Yasuo
Recent Advances in Actinide Science, p.448 - 450, 2006/06
A pyrochemical process for the metallurgical treatment of the spent nuclear fuels contains the Cd-distillation step, where Pu is recovered as a residue. For understanding this Cd-ditillation behavior the samples of PuCd +PuCd
+PuCd and PuCd
and PuCd +PuCd
+PuCd were prepared and Knudsen-cell mass-spectrometric measurements were carried out to determine the vapour pressures of Cd(g) over these samples. Further,the thermodynamic quantities of PuCd
were prepared and Knudsen-cell mass-spectrometric measurements were carried out to determine the vapour pressures of Cd(g) over these samples. Further,the thermodynamic quantities of PuCd and PuCd
and PuCd were evaluated from these vapour pressures.
were evaluated from these vapour pressures.

Nakajima, Kunihisa; Arai, Yasuo; Yamashita, Toshiyuki
Journal of Physics and Chemistry of Solids, 66(2-4), p.639 - 642, 2005/02
Times Cited Count:2 Percentile:12.62(Chemistry, Multidisciplinary)PuCd intermetallic compound was prepared by heating pure Pu and Cd metals at about 950K. PuCd
intermetallic compound was prepared by heating pure Pu and Cd metals at about 950K. PuCd was found to be a prototype of CdI
was found to be a prototype of CdI by means of powder X-ray diffractometry. Mass-spectrometric experiment was performed in the temperature range of 650-770K. It was found that the vapor pressures of Cd over PuCd
by means of powder X-ray diffractometry. Mass-spectrometric experiment was performed in the temperature range of 650-770K. It was found that the vapor pressures of Cd over PuCd +Pu were three to five orders of magnitude lower than those over Cd in this temperature range. From these vapor pressures, Gibbs free energy of formation of PuCd
+Pu were three to five orders of magnitude lower than those over Cd in this temperature range. From these vapor pressures, Gibbs free energy of formation of PuCd was evaluated.
was evaluated.
Nakajima, Kunihisa; Arai, Yasuo
Journal of Nuclear Materials, 317(2-3), p.243 - 251, 2003/05
Times Cited Count:3 Percentile:25.79(Materials Science, Multidisciplinary)The Knudsen effusion mass-spectrometric measurement of pure UO (s) are carried out at 1673, 1773 and 1873K to evaluate
(s) are carried out at 1673, 1773 and 1873K to evaluate  G
G
 (UO
(UO ,g) as well as to measure the partial pressures of UO
,g) as well as to measure the partial pressures of UO (g) and O
(g) and O (g) over UO
(g) over UO (s) as function of the O/U ratio. It was found that the partial pressures of O
(s) as function of the O/U ratio. It was found that the partial pressures of O (g) over UO
(g) over UO (s) almost agree with the experimental data reported in the past and the values derived from the empirical equation given by Nakamura and Fujino. Further, it was found that the values of
(s) almost agree with the experimental data reported in the past and the values derived from the empirical equation given by Nakamura and Fujino. Further, it was found that the values of  G
G
 (UO
(UO ,g) obtained in this study are in good agreement with the recommended values.
,g) obtained in this study are in good agreement with the recommended values.
Nakajima, Kunihisa; Omichi, Toshihiko*; Arai, Yasuo
Journal of Nuclear Materials, 304(2-3), p.176 - 181, 2002/08
Times Cited Count:3 Percentile:23.41(Materials Science, Multidisciplinary)The oxygen potentials of two type urania-yttria solid solutions, one is the solid solution expressed as nearly U Y
Y O
O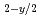 and the other is nearly stoichiometric solid solution, are investigated by use of a mass spectrometer combined with a Knudsen cell. The oxygen potentials for the nearly stoichiometric specimens are much higher than those for pure urania with similar stoichiometry. On the other hand, the oxygen potentials for fully reduced specimens almost agree with those for pure urania having the same oxygen deficiencis.
and the other is nearly stoichiometric solid solution, are investigated by use of a mass spectrometer combined with a Knudsen cell. The oxygen potentials for the nearly stoichiometric specimens are much higher than those for pure urania with similar stoichiometry. On the other hand, the oxygen potentials for fully reduced specimens almost agree with those for pure urania having the same oxygen deficiencis.
Maeda, Atsushi; ; Omichi, Toshihiko
Journal of Alloys and Compounds, 179, p.L21 - L24, 1992/00
Times Cited Count:17 Percentile:92.86(Chemistry, Physical)no abstracts in English
; ; ;
JAERI-M 7572, 20 Pages, 1978/03
no abstracts in English

Nakajima, Kunihisa
no journal, ,
Results on the thermodynamic study of gaseous CsBO will be presented as a keynote speech of the 64th annual conference on Mass Spectrometry. Gaseous cesium metaborate, CsBO
will be presented as a keynote speech of the 64th annual conference on Mass Spectrometry. Gaseous cesium metaborate, CsBO (g), is predicted to be the main cesium vapor species in severe light water reactor accidents according to the thermodynamic analysis, which treats chemical reactions with B
(g), is predicted to be the main cesium vapor species in severe light water reactor accidents according to the thermodynamic analysis, which treats chemical reactions with B C. But quality of the thermodynamic data of CsBO
C. But quality of the thermodynamic data of CsBO (g) used in this thermodynamic analysis is regarded as poor. Thus, Knudsen-effusion mass-spectrometric measurement of CsBO
(g) used in this thermodynamic analysis is regarded as poor. Thus, Knudsen-effusion mass-spectrometric measurement of CsBO was performed to obtain reliable thermodynamic quantities of CsBO
was performed to obtain reliable thermodynamic quantities of CsBO (g). The standard enthalpy values of formation of CsBO
(g). The standard enthalpy values of formation of CsBO (g),
(g), 

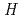
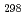
 (CsBO
(CsBO ,g), were obtained by the second and third law treatments and found to be -700.7
,g), were obtained by the second and third law treatments and found to be -700.7 10.7kJ/mol and -697.0
10.7kJ/mol and -697.0 10.6kJ/mol. These two values agree within the error limits and the evaluated thermodynamic data of CsBO
10.6kJ/mol. These two values agree within the error limits and the evaluated thermodynamic data of CsBO (g) are considered to be reliable.
(g) are considered to be reliable.